Hello, fellow mining engineers! Today, we’re diving into a critical and potentially hazardous chemical phenomenon in mining operations—the natural weathering of iron sulfide and its dangerous interaction with ammonium nitrate that can lead to explosions. This isn’t just a theoretical exercise from a lab; it’s a real-world safety concern that demands our attention. Let’s break down the process, understand the mechanisms, and explore how to mitigate the risks.
Natural Weathering: The Oxidation of Iron Sulfide
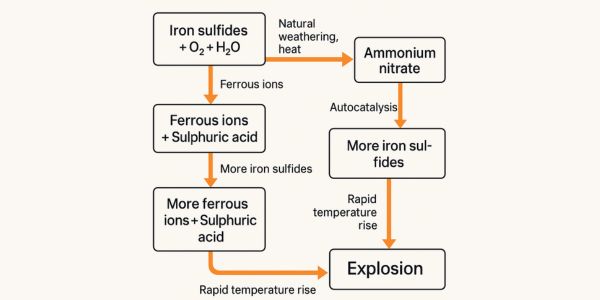
In mining environments, iron sulfide—often in the form of pyrite, also known as “fool’s gold”—is commonly found in rock fissures, blast residue piles, open pits, or abandoned mine shafts. When exposed to air and moisture, it undergoes a natural oxidation reaction, producing ferrous ions and sulfuric acid. The chemical process can be summarized as: Iron Sulfide + Oxygen + Water → Ferrous Ions + Sulfuric Acid
This reaction is exothermic, meaning it releases heat. Under certain conditions, localized temperatures can rise to hundreds of degrees Celsius. This is particularly notable in drill holes, where the phenomenon is often called a “hot blast hole”—a situation where the area experiences abnormal temperature spikes due to the reaction. This heat buildup sets the stage for more dangerous reactions down the line.
The Hazardous Interaction with Ammonium Nitrate
Ammonium nitrate (AN), a key component in common mining explosives like ANFO (ammonium nitrate fuel oil), is generally stable under normal conditions. However, when the byproducts of natural weathering—ferrous ions and sulfuric acid—come into contact with AN, a troubling chemical reaction begins: Ammonium Nitrate + Iron Sulfide + Ferrous Ions + Sulfuric Acid → Nitric Oxide + Ferric Ions + Heat
This reaction is self-catalytic, meaning the products it generates (like nitric oxide and ferric ions) further accelerate the reaction, creating a positive feedback loop. The heat released during this process adds to the thermal stress on the surrounding environment, threatening the stability of ammonium nitrate. Think of it as a small glitch in a system that snowballs into a full-scale failure if left unchecked.
Chain Reaction: Nitric Oxide and Iron Sulfide
The byproducts don’t stop there. Nitric oxide and ferric ions produced in the previous step continue to react with additional iron sulfide present in the area: Iron Sulfide + Nitric Oxide + Ferric Ions → Ferrous Ions + Sulfuric Acid
This step also releases heat, and the reaction rate increases as catalytic substances accumulate. Initially, the reaction progresses slowly during what’s known as an “induction period,” where temperature changes may be barely noticeable. But once the concentration of catalytic agents reaches a critical threshold, the reaction rate surges dramatically, leading to significant heat output. This sudden acceleration paves the way for catastrophic consequences.
The Final Threat: Ammonium Nitrate Explosion
As temperatures climb to around 220°C or even lower, ammonium nitrate can rapidly decompose and trigger an explosion: Ammonium Nitrate + Heat (≤220°C) → Explosion
What begins as a slow natural weathering process can spiral into an uncontrollable explosion. Each step in this reaction chain builds up heat and reactive compounds, eventually pushing ammonium nitrate past its stability threshold. This risk is especially severe in mining settings, where localized high temperatures in blast holes or stockpiles often go undetected until it’s too late.
Reaction Pathway Summary
Here’s a simplified breakdown of the reaction chain to help visualize the progression: - Stage 1: Iron Sulfide + Oxygen + Water → Ferrous Ions + Sulfuric Acid (Natural Weathering, Exothermic) - Stage 2: Addition of Ammonium Nitrate → Nitric Oxide + Ferric Ions + Heat (Self-Catalytic Reaction) - Stage 3: More Iron Sulfide Reacts → Additional Ferrous Ions + Sulfuric Acid (Reaction Accelerates) - Stage 4: Temperature Spikes → Ammonium Nitrate Decomposes → Explosion
This sequence shows how a seemingly benign natural weathering reaction can cascade into a disastrous event if not managed properly.
Why This Matters to Mining Engineers
For engineers in mining or explosives management, understanding this reaction mechanism is not just academic—it’s essential for safety. According to reports from reputable sources like the Australian Mining Safety Journal, several incidents over the past decade involving reactive ground and ammonium nitrate have been directly linked to this chemical reaction chain. Ignoring these risks can result in loss of life, equipment damage, and operational downtime.
From an engineering perspective, this phenomenon also serves as a reminder that the stability of any system must account for external variables. Whether in mining or other technical fields, small initial issues can escalate into systemic failures if not addressed early.
Preventive Measures and Safety Recommendations
To minimize the risk of such explosions, mining engineers can implement the following strategies: - Assess Reactive Ground: Regularly analyze rock samples for iron sulfide content. If high levels of pyrite are detected, treat the area with heightened caution and consider isolation measures. - Ammonium Nitrate Storage and Handling: Store AN away from reactive materials and maintain cool, dry conditions to prevent contact with weathering byproducts. - Temperature Monitoring: Deploy temperature sensors in blast holes and stockpile areas to detect abnormal heat spikes in real time, allowing early intervention for “hot blast holes.” - Personnel Training: Ensure on-site teams are trained to recognize signs of reactive ground and runaway reactions with ammonium nitrate, and are familiar with emergency response protocols.
Conclusion: Mitigating Risks with Science
The interaction between iron sulfide’s natural weathering and ammonium nitrate is a serious safety hazard in mining operations. From the initial slow exothermic reaction to the potential for a full-scale explosion, this process underscores the dangers of chemical interactions between natural elements and man-made materials. As engineers, our responsibility is to use scientific understanding and rigorous management practices to prevent such risks from materializing.
I hope this post provides valuable insights for your mining safety practices. If you’ve encountered similar hazards or have additional experiences to share, feel free to drop them in the comments. Let’s work together to keep our mine sites safe and operational!
comments (0)
please login to comment